CAR T Cell Therapy
Overview
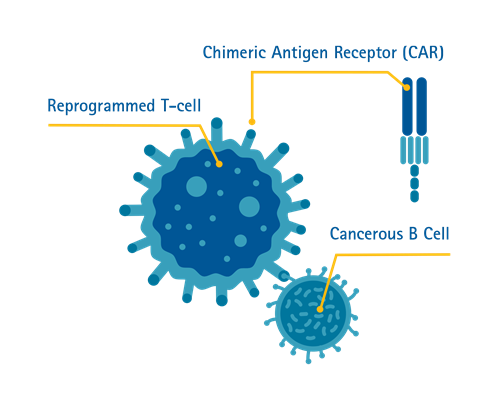
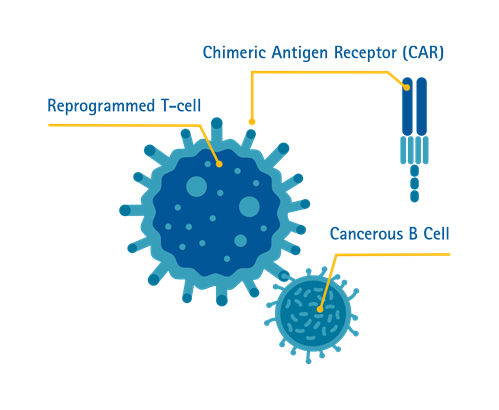
What is chimeric antigen receptor (CAR) T cell therapy?
T cells or T lymphocytes are a type of white blood cell in the immune system. T cells have the ability to recognize abnormal cells or virus-infected cells in the body and then destroy these abnormal cells.
However, T cells can sometimes fail to recognize or ignore these threats in the body, such as in the case of cancer.
Chimeric antigen receptor (CAR) T cell therapy is a method immunotherapy in which T cells are taken from the patient's blood and modified in the laboratory to enable the T cells to identify and kill specific cancer cells. The modified T cells are then returned to the patient. Once back in the patient's body, the modified T cells will be able to detect the cancer cells and kill the cancer by harnessing the body's own immune response.
Singapore is the first country in Southeast Asia to adopt this treatment method^.
^Source: CNA
What diseases can this method treat?
CAR T cell therapy is particularly effective for patients diagnosed with certain types of acute lymphoblastic leukemia (ALL) high-grade and malignant recurrence non-Hodgkin's lymphoma relapsed diffuse large B-cell lymphoma (DLBCL), especially when at least two prior treatment regimens have failed to produce the desired results.
Which patients are eligible?
Selected patient groups eligible for CAR T cell therapy include:
- Pediatric and young adult patients 2 to 25 years of age with refractory B-cell acute lymphoblastic leukemia (ALL) who relapsed or refractory to allogeneic therapy after stem cell transplantation.
- Adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have not responded to at least one or two standard treatments.
- Adults with relapsed or refractory follicular lymphoma (FL) who have not responded to at least two standard therapies.
- Patients with intracranial hypertension or loss of consciousness
- Patient with respiratory failure
- Disseminated intravascular coagulation patient
- Patients with uncontrolled active sepsis or infection
CAR T Cell Therapy
How is this method performed?
First, the patient will be examined and have tests done to determine if CAR T cell therapy is an appropriate treatment option for the disease and to ensure that the patient is healthy enough for treatment.
Step 1. Collect T cells
White blood cells, including T cells, are taken from the patient's blood using a procedure called leukapheresis. During the procedure, two intravenous (IV) lines are placed in the patient: blood is removed through one line, to separate and extract the white blood cells, while the remaining blood is returned to the patient's body through the second line.
The patient will be asked to lie or sit in a recliner during the procedure.
Step 2. Creating CAR T cells
Once the white blood cells have been extracted, the T cells are separated and sent to the lab for modification. Doctors modify them by adding a specific chimeric antigen receptor (CAR) gene to the T cells, thus changing them into CAR T cells. These cells are then cultured and multiplied in the lab.
Under normal circumstances, CAR T cell therapy can take 2-3 weeks to produce the required number of CAR T cells.
Step 3. Reinfusion of CAR T cells
Once enough CAR T cells have been produced, they are returned to the hospital for infusion into the patient.
A few days before the CAR T cell infusion, the patient may receive valence to reduce the number of other immune cells in the body and prepare the body to receive the CAR T cells. This gives the newly infused CAR T cells a better chance of being “activated” against the cancer cells. In general, the chemotherapy is less intense to ensure that there are enough cancer cells left for the CAR T cells to effectively “activate.”
As CAR T cells begin to bind to cancer cells in the body, they begin to increase in number and kill more and more cancer cells.
Step 4. Recovery
Patients treated with CAR T-cell therapy will have an early recovery period of about 6-8 weeks. During this time, patients will be monitored for side effects and treatment response.
What is the recovery process like?
Recovery usually takes 2-3 months from the CAR T cell infusion. Patients will be hospitalized for the first 2-3 weeks to recover from side effects before being discharged.
After discharge from the hospital, patients will need to have regular outpatient visits to monitor side effects and clinical treatment responses.
Side Effects of CAR T Cell Therapy
What are the side effects?
A common side effect of CAR T cell therapy is cytokine release syndrome (CRS), a multisystem illness caused by the action of the CAR T cells to activate and kill cancer cells.
Side effects of CRS include:
- High fever and chills
- Shortness of breath
- Nausea, vomiting and/or diarrhea
- Feeling dizzy and lightheaded
- Headache
- Rapid heart rate
- Tired
- Muscle and/or joint pain
CRS may occur several weeks after infusion, but usually occurs within two weeks of infusion. The severity of CRS does not correlate with response to CAR T-cell therapy.
Another common side effect is immune effector cell-associated neurotoxic events (ICANS), which affect the central nervous system.
CRS and ICANS are highly treatable side effects that can be managed by a trained clinical care team.
Benefits and Difficulties
What are the benefits and challenges?
CAR T-cell therapy offers leukemia patients a potentially life-saving treatment option if their disease is not controlled by valence standard, targeted therapy or bone marrow transplant
However, as it is a relatively new field of cell therapy, there are several challenges to consider such as selecting patients who will benefit from this treatment, how well it suits their health, the timing of cell collection, logistical concerns, and the risk-benefit ratio of the treatment for each patient.
-------------------------------------------------------------------------------------------
👉 Contact SunCare for medical support and advice as well as professional private jet transportation services 🇸🇬 SUNCARE PTE. LTD SINGAPORE
🏠 Add: 10 Anson Road, #10-11 International Plaza, Singapore 079903
☎️ Hotline: +65 96727717 (Dr. Lien Minh - Director) Zalo, Viber
📨 Email: suncarehealth@gmail.com